Genetic engineers and researchers alike have explicitly shared their affinity towards the gene-editing tool called CRISPR–Cas9. This device alters targeted genes effortlessly, thereby permitting an executable approach to developing treatments and cures. There is, however, a major limitation to Cas9 in terms of rectifying an isolated DNA letter in a gene.
Genetic engineers and researchers alike have explicitly shared their affinity towards the gene-editing tool called CRISPR–Cas9. This device alters targeted genes effortlessly, thereby permitting an executable approach to developing treatments and cures. There is, however, a major limitation to Cas9 in terms of rectifying an isolated DNA letter in a gene.
To address and remedy this situation, David R. Liu, a chemical biologist at Harvard University in Cambridge, Massachusetts, along with his fellow Harvard researchers like Alexis Komor, took the initiative to engineer the Cas9, facilitating the unique ability to modify a single DNA letter within the given gene in a pinpoint fashion.
This will not only ameliorate the success rate gene edits but also endow doctors and researchers with the power of modelling human ailments and consequentially develop therapeutic methodologies for them.
In David Liu’s words, “The majority of disease-associated human genetic variants are point mutations. But current genome methods correct point mutations much less efficiently and much less cleanly than we can disrupt a gene.”
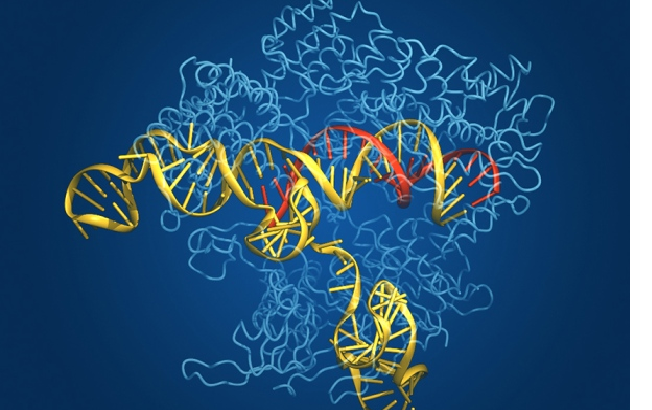
Although the advent of Cas9 has been a breakthrough in the realm of Genetic Engineering, this enzyme comes with its own precincts and shortcomings. Cas9 directs its focus to a single site in the genome, guided by an RNA molecule.
The strands of the DNA mirroring the guiding RNA molecule are then severed at the targeted spot. This causes the procedure to be executed in a slipshod manner as sometimes addition or omission of DNA letters takes place while the cells endeavour to mend the break. The corollary of this action is a defunct gene.
Hence, this prompted David Liu and his team to contrive a method of pruning the genome while preventing any breakage. This was achieved by incapacitating the Cas9 enzyme, thereby eradicating the possibility of it cutting the DNA, and then annexed it to another enzyme which can chemically switch one DNA letter to another. The incapacitated Cas9 was then be navigated by a guiding RNA to reach its target point within the genome. Thus, the DNA sequence altered without pruning the helix on the whole.
Liu opines, “There are hundreds of other disease-associated mutations that could be corrected using a C to U switch.” His consortium of researchers is not endeavouring to augment the reach across alternative point mutations.
This methodology has the potential of incorporating alternative enzymes to Cas9, such as Cpfl. This is deemed more suitable for inducing sequences into genes. Nature has published two paper, shedding light on the functionality of Cpf1. According to these papers, Cpfl can shape-shift once it is appended to a guiding RNA and cis capable of slicing both DNA as well as RNA effectively. One shows how the enzyme changes shape when bound to its guide RNA; the other describes a Cpf1 enzyme capable of cleaving both DNA and RNA.
A bioengineer at Stanford University in California by the name Stanley Qi, says, “This method could be much simpler than the old CRISPR method, which required inserting a DNA template that is prone to degradation and was sometimes even toxic to cells. This avoids a major barrier. It really expands the scope of CRISPR’s applications.”